A Fusion of Science, Medicine, and Nature
The patent pending Boscumin™ complex has been clinically developed to deliver 29X more active natural anti-inflammatories‡ to your body and joints and increase your mobility by over 300%†.
Try risk freeOur Clinical Study: Effectiveness
Our clinical studies show that Boscumin increases mobility and comfort by over 300%†.
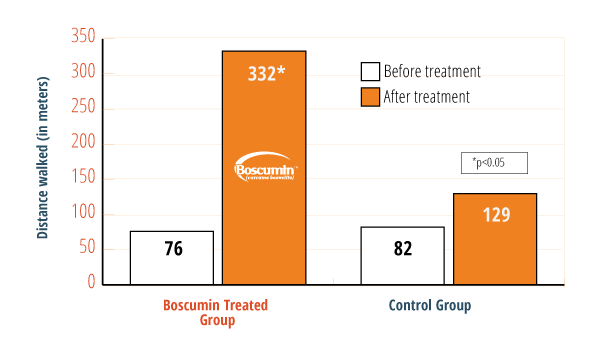
Study Highlights
After 3 months, those taking the supplement could walk 330% farther† than those in the placebo group.
50% improvement in pain and stiffness† when compared to the placebo control group (based on WOMAC scores).
Results showed a 63% reduction in NSAID† (Advil, etc.) or acetaminophen (Tylenol) use.
16x reduction in inflammatory markers† in the blood.
The Boscumin Difference: Absorption
Over 8000 studies tout the benefits of turmeric and boswellia. However, food and supplements contain a very small amount of the active compounds, curcumin and boswellic acid.
More importantly, both compounds are very poorly absorbed by our bodies, leading to such low concentrations as to show little to no health benefits. In fact, studies show that 61% of name brand curcumin based supplements are not absorbed by the body at all.
Boscumin’s physician-developed pharmaceutical-grade formula was designed to specifically overcome these challenges.
Boscumin's innovative technology fuses curcumin and boswellic acid to fat soluble lecithin for improved stability and 29x more absorption‡.
try nowOur Clinical Study: Absorption
The Boscumin complex has been clinically developed to deliver significantly more curcumin and boswellic acids to your joints.
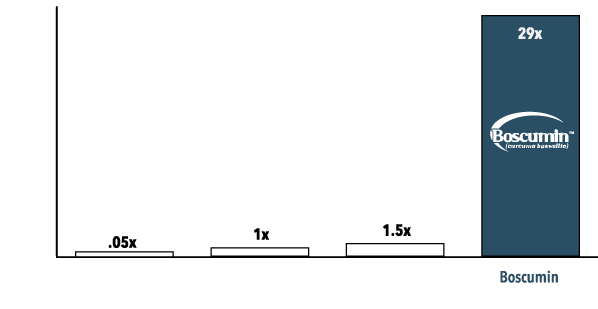
Study Highlights
Total curcuminoid absorption 29x higher‡ than standard curcumin.
35x Increase in KBA and AKBA (boswellic acids)‡ concentrations in brain tissue.
50x to 60x increase‡ in curcumin metabolites.
The Boscumin Difference: Sourced from Plants not Petroleum
It is estimated that up to 40% of curcumin supplements are made from petroleum, instead of from plant based sources.
Boscumin in the only product that goes through rigorous radiocarbon testing to guarantee its natural origins.
* Your bottle of Boscumin will always contain a QR code allowing you to track its natural origin from plant to pill.
Experience the boscumin differenceThe Most Clinically Proven Natural Joint Remedy
The ingredients in Boscumin have been clinically proven to provide:
Less pain
Greater physical function
Less stiffness
Greater overall Quality of Life
Reduced muscle soreness
16x reduction in C-Reactive Protein (a specific inflammation indicator)†
Review Our Clinical Trial Library
1. Allegri P., et al. Clin Ophthalmol, 2010. 4: p. 1201-6.
2. Antiga A., et al. BioMed Research International, vol. 2015, Article ID 283634.
3. Appendino G., et al. Panminerva Med, 2011. 53 [3 Suppl 1]: p. 43-9.
4. Belcaro G., et al. Altern Med Rev, 2010. 15 [4]: p. 337-44.
5. Belcaro G., et al. Eur Rev Med Pharmacol Sci, 2014. 18 [24]: p. 3959-63.
6. Belcaro G., et al. Panminerva Med, 2010. 52 [2 Suppl 1]: p. 55-62.
7. Belcaro G., et al. Phytother Res, 2014. 28 [3]: p. 444-50.
8. Di Pierro F., et al. J Pain Res. 2013, 6, 201-205.
9. Di Pierro F. and R. Settembre J Pain Res, 2013. 6: p. 497-503.
10. Drobnic F., et al. J Int Soc Sports Nutr, 2014. 11: p. 31.
11. Ferguson, Jessica JA, et al. Metabolism [2017].
12. Ferrara T., et al. Eur Rev Med Pharmacol Sci. 2015 Oct;19(19):3757-62.
13. Franceschi F., et al. Eur Rev Med Pharmaco Sci. 2016; 20: 762-766.
14. Franceschi F., et al. Eur Rev Med Pharmacol Sci. 2016 Oct;20(19):4156-4161.
15. Golombick T., et al. Journal of Cancer Therapy, 2015, 6 [7], 566-571.
16. Kayabasi U., et al. Int J Ophthalm Clin Res 2014 1:002.
17. Lazzaro F., et al. GIOT 2014 Jul(40):249–257.
18. Lazzaro F., et al. GIOT 2014 May(40): 141-50.
19. Lazzaro F., et al. GIOT 2014(40):1–10.
20. Lazzaro F., et al. GIOT 2014 41:80–89.
21. Ledda A., et al. Panminerva Med, 2012. 54 [1 Suppl 4]: p. 17-22.
22. Maccio A., et al. Nutrition, 2015. 31[1]: p. 239-43.
23. Mazzolani F. Clin Ophthalmol, 2012. 6: p. 801-6.
24. Mazzolani F. and S. Togni Clin Ophthalmol, 2013. 7: p. 939-45.
25. Mazzolani F. and S. Togni, et al. Minerva Ophthalmol, 2016. 58: p 1-5.
26. Pajardi G., et al. Evid Based Complement Alternat Med, 2014. 2014: p. 891310.
27. Panahi Y., et al. J. Cardiovasc Pharmacol. 2016; 68: 223 -229.
28. Panahi Y., et al. Drug Res [Stuttg]. 2017 Apr; 67 [4]: 244-251.
29. Panahi Y., et al. J Functional Food 2014 [6] 615–622.
30. Panahi Y., et al. Phytother Res, 2014. 28 [10]: p. 1461-7.
31. Pellegrini L., et al. Eur Rev Med Pharmacol Sci. 2016 Jun;20(12):2695-700.
32. Riva A., et al. Eur Rev Med Pharmaco Sci. 2017; 21: 1684:1689.
33. Riva A., et al. Phytomedicine. 2016 Nov 15;23(12):1375-1382.
34. Riva A., et al. Eur Rev Med Pharmacol Sci 2017(21):5258-5263
35. Sciberras J.N., et al. J Int Soc Sports Nutr, 2015. 12 [1]: p. 5.
36. Serpe R., et al. 6th Cachexia Conference, Milan, Italy, December 8–10, 2011.
37. Soldà C., et al. International Journal of Pharmacology, Phytochemistry and Ethnomedicine, Vol 2, 2017.
38. Steigerwalt R., et al. Panminerva Med, 2012. 54 [1 Suppl 4]: p. 11-6.
39. Szymanski M.C., et al. J Appl Physiol 2017. doi: 10.1152/japplphysiol.00515. 2017.
40. Tenero L., et al. Allergy Asthma Proc. 2016; 37:e8-e13.
41. Watson R.R., Preedy V.R., Bioactive Foods as Dietary Interventions for Arthritis and Related Inflammatory Diseases, Ch. n.5, Appendino, Togni, p. 66-81.
42. Watson R.R., Foods and Dietary Supplements in the Prevention and Treatment of Disease in Older Adults, Elsevier Science, 2015, p. 16.

 read the clinical study
read the clinical study